Regulatory affairs
Regulatory affairs
Our international teams and project managers have acquired high technical know-how and regulatory skills at national and regional level.
Thanks to our skilled teams as well as the reliable connections that we have with the local regulatory departments, we are able to identify the needs and special requirements for the registration of agricultural commodities.
Together with reliable local experts, BIOTEK AAA is able to produce registration dossiers.
BIOTEK AAA’s teams are fully committed to build protocols and run trials which are fully GEP or GLP compliant according to local regulations. Our continuous technological and regulatory watch are a product registration success guarantee for our international partners and customers.

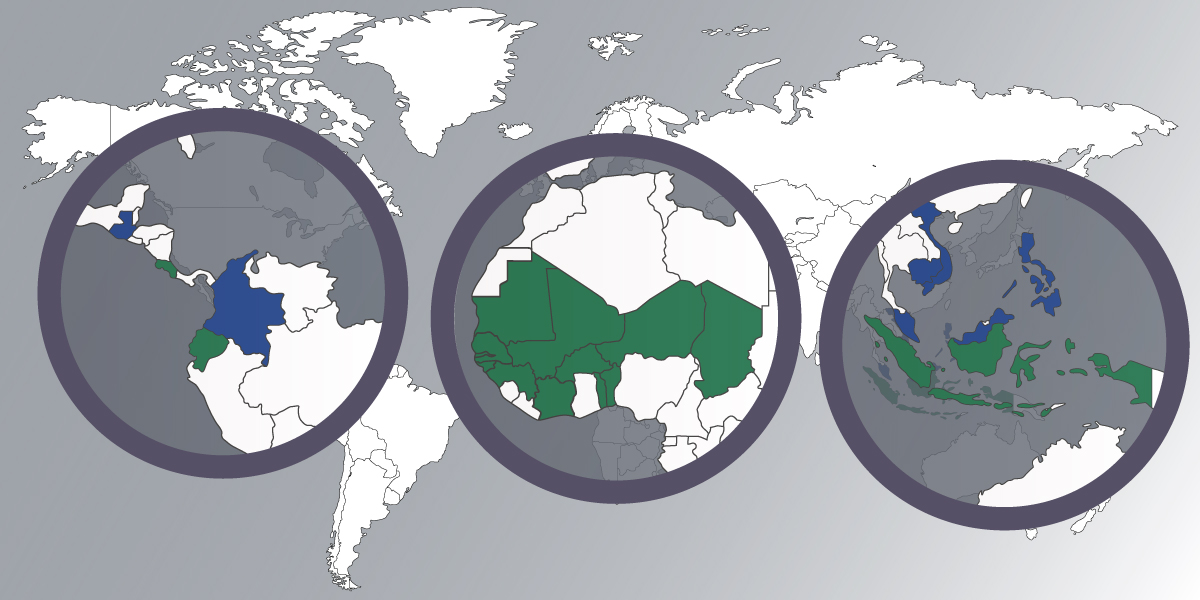
List of countries where we can carry out regulatory projects
|
|
|---|
|
|
|---|
Please find below the list of countries where we can carry out regulatory projects:
A- Africa: Benin, Burkina Faso, Cape Verde, Ivory Coast, Gambia, Guinea, Guinea-Bissau, Mali, Mauritania, Niger, Senegal, Chad, Togo
We can hold the registration in all these countries through our subsidiary Biotek West Africa
B- Latin America: Colombia, Costa Rica, Ecuador, Guatemala.
We can hold the registration in Costa Rica and Ecuador
C- Southeast Asia: Indonesia, Malaysia, Philippines, Thailand, Vietnam
We can hold the registration in Indonesia
Contact us

116 Rue Paul Doumer
10 300 Sainte Savine
France
contact@biotek-aaa.com



